Microwaves as an Energy Transfer Method in Chemical Processes
In recent years, the research and implementation of microwave technology have accelerated across various industrial fields. Commonly known for heating via microwave ovens, microwaves are a type of electromagnetic wave also used in communications and sensors, possessing an extensive range of applications. Among these, their use as an energy transfer method in chemical processes is garnering significant attention. Leveraging characteristics distinct from conventional heat sources, microwave technology is anticipated to enable highly efficient reactions and energy conservation [1].
This article explains how microwaves are utilized in chemical processes, detailing their fundamental principles, application examples, technological advantages, and challenges. Specifically, it covers microwave heating mechanisms, characteristics of selective heating, practical examples, advancing control technologies, and future prospects. Unraveling these points helps to provide insights into microwave chemical processes and their practical applications.
Properties of Microwaves and Heating Mechanisms
1. What are Microwaves?
Microwaves are a type of electromagnetic wave with frequencies ranging from 300 MHz (0.3 GHz) to 300 GHz, corresponding to wavelengths of approximately 1 meter to 1 millimeter. They are part of the “radio wave” spectrum used for television broadcasting and mobile phone communications, applied in a wide array of uses.
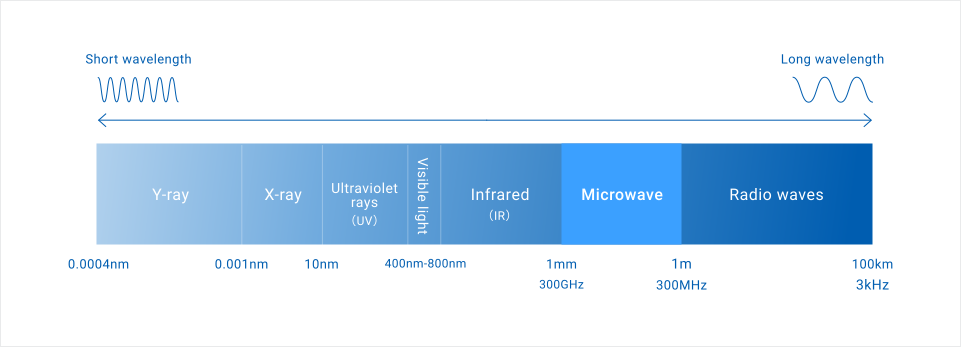
The adoption of this frequency band as a “heat source” stems from advancements in radar technology during World War II. Post-war, microwave heating technology, a byproduct of radar research, was adapted for civilian use, leading to the invention and commercialization of the microwave oven by the Raytheon Company (patented in the 1940s). The development spurred the widespread adoption of microwave heating, for industrial applications as well as household kitchens[2].
2. Microwave Heating Mechanisms
When microwaves irradiate a substance, molecules within it rapidly rotate or vibrate in response to the oscillating electromagnetic field. Polar molecules, such as water, rotate according to the microwave’s electric field, generating thermal energy through intermolecular friction [3]. This is the basic mechanism of microwave heating. While commonly known as “dielectric heating,” other mechanisms exist: “magnetic loss heating,” resulting from the interaction between the magnetic field component and the substance, and “conductive loss (Joule heating),” occurring when microwaves act on conductive materials. However, research into these non-dielectric heating mechanisms is relatively limited, and a detailed understanding and expansion of their applications is currently under investigation. A deeper understanding of these mechanisms could further broaden the applicability of microwave technology. Regardless of the mechanisms, the key feature of microwave heating lies in its efficient and selective energy transfer through mechanisms different from conventional heating, forming a core aspect of microwave chemistry.
Furthermore, the ease with which different substances absorb microwaves varies (differing dielectric loss factors), allowing for the advantage of “selective heating.” For instance, materials highly transparent to microwaves, like glass or polypropylene, experience minimal temperature rise, whereas polar molecules, such as water or ionic solutions, heat up significantly. By skillfully utilizing this property, it becomes possible to efficiently heat only the necessary substances within a reaction system, thereby suppressing unwanted side reactions.
3. Internal Heating and Rapid Response
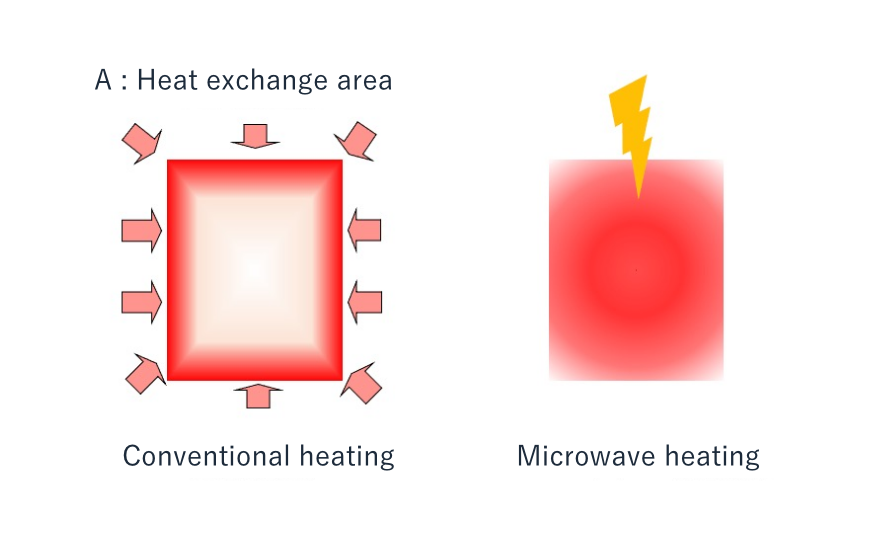
Microwave heating induces heat generation directly within the substance rather than from the outside, resulting in an extremely fast heating response. Conventional heating methods require time for heat conduction, leading to delays in reaching temperature and temperature non-uniformity, which can reduce reaction efficiency and cause side reactions.
In contrast, microwaves can be applied instantaneously when needed, allowing for rapid reaction initiation and high flexibility in switching heating on and off, improving overall process controllability. This high responsiveness is advantageous not only in batch reactions but also in continuous processes.
Microwaves offer a highly flexible heating method for requirements such as precise chemical reaction control, suppression of side reactions, and management of temperature record . While this characteristic is seen in food heating, in chemical processes, the goal is to build highly efficient reaction systems through more precise control and selective heating, advancing the field of chemistry microwave applications.
The Role Microwaves Should Play in the Chemical Industry
1. New Value Brought by Microwaves
The chemical industry often involves processes requiring high temperatures, high pressures, or prolonged heating, entailing substantial energy costs and environmental burdens. Against this backdrop, microwaves are gaining attention as an innovative heat source capable of executing these processes rapidly and efficiently.
Particularly in countries like Japan, which rely on foreign fossil fuels, maximizing output from limited primary energy resources is directly linked to maintaining industrial competitiveness. The high energy conversion efficiency and selective heating characteristics of microwaves can potentially bring significant value to the chemical industry, aiming to achieve energy savings and decarbonization.
2. Comparison with Conventional Equipment
Heating equipment traditionally used widely in the chemical industry, such as jacketed reactors and heat exchangers, is primarily based on designs that conduct heat from the outside. Such methods require time to heat the entire reaction system, inevitably involving equipment upsizing and energy loss. Conversely, microwave heating utilizes heat generation within the substance, fundamentally changing the premises of equipment design. This results in the following advantages:
- Rapid Startup: Microwaves can be irradiated and heat generated instantly when needed, eliminating unnecessary waiting times.
- Uniform Temperature Distribution: While conventional methods tend to create localized high-temperature zones near walls or the bottom, microwaves distribute energy throughout the interior, suppressing temperature non-uniformity.
- Miniaturization and Modularization: Eliminating the need for large heating furnaces and long pipelines enhances process scalability. However, new design challenges, such as shielding design to handle high-power microwaves safely and efficiently, and the maintenance and replacement costs of oscillation sources (magnetrons or solid-state amplifiers), also arise. Therefore, applying microwave heating to industrial equipment requires a comprehensive evaluation considering both technological advantages and operational requirements. This evaluation is crucial when considering the chemical technology of radiation heat transfer via microwaves.
3. Specific Advantages in the Chemical Industry
Introducing microwave technology is expected to yield the following specific effects in the chemical industry:
- Improved Productivity through Reduced Reaction Times: Significantly shortening the processing time per batch can increase throughput with existing equipment, improving plant utilization and line efficiency [4].
- Energy Savings and CO₂ Emission Reduction: Since microwaves can selectively heat only the necessary parts, heat loss is minimized. Furthermore, achieving faster and more efficient chemical reactions contributes to reducing overall energy consumption and environmental impact [5].
- Enhanced Product Quality: Conventional heating often leads to side reactions and thermal degradation, causing issues like product discoloration and property variations. Microwave heating, enabling rapid and uniform heating, is advantageous for quality aspects such as suppressing byproducts and controlling molecular weight [6]. In recent years, large-scale plants incorporating microwave technology have started operation in Japan, with reported cases of significant energy cost reductions compared to conventional technologies [7]. The accumulation of such achievements is expected to further promote the adoption of microwave technology in the industry.
Promoting Chemical Reactions with Microwaves
1. Mechanisms for Enhancing Reaction Rates
One of the most significant advantages of microwave heating is its ability to dramatically increase the speed of chemical reactions. Conventional heating via thermal conduction takes time to reach the reaction temperature, during which temperature non-uniformity and gradients can occur, potentially leading to uneven reaction progress or promoting side reactions.
Conversely, microwaves generate heat directly within the target substance, allowing the entire reaction system to be heated uniformly in a short time, enabling a rapid transition to a high-energy state. Such heating allows reactions requiring activation energy to proceed simultaneously, increasing reaction rates.
Furthermore, some reports suggest that microwave irradiation itself may produce “non-thermal effects” beyond simple heating. Examples include molecular orientation effects and changes in the reaction field due to the electric field, though these phenomena are still under debate and await systematic verification [8].
2. Specific Examples
Specific examples demonstrating the effectiveness of microwave heating include:
- Esterification Reaction (Organic Synthesis): An esterification reaction that conventionally took about 1 hour was reported to be completed in just 12 minutes with microwave irradiation, achieving a conversion yield of 90% or more [9]. The improvement is attributed to reaching the reaction temperature quickly and heating the entire system uniformly.
- Styrene Polymerization (Polymer Synthesis): Research by a company indicated that using microwave irradiation reduced the polymerization time of styrene to about one-third of the conventional method, and control over the degree of polymerization became easier [10]. The improvement is presumed to be due to the accelerated reaction from efficiently heating and mixing monomers and radical species.
- Dehydration Condensation Reaction (Water Byproduct): In reactions producing water as a byproduct, the high microwave absorption of water molecules can be utilized to rapidly remove water from the reaction system [11]. Such removal can shift the equilibrium towards the products, potentially leading to improved yields. These achievements are progressing beyond laboratory scale to verification in industrial plants. If microwaves become widely adopted as a technology to dramatically shorten the rate-limiting steps of entire processes, they are expected to contribute to productivity improvements in the chemical industry.
Mechanism of Energy Transfer by Microwaves
1. Interaction between Microwaves and Matter
Microwaves are a form of electromagnetic wave that interacts with matter primarily through three mechanisms: dielectric loss, conduction loss, and magnetic loss.
For example, substances rich in polar molecules like water exhibit high dielectric loss. These molecules undergo rotational motion in response to the microwave’s electric field, generating heat through friction. This is the most common phenomenon in microwave heating. In highly conductive materials like graphite or carbon fibers, microwaves induce electric currents, generating heat through resistance heating (Joule heat). This effect is known as conductive loss. Conversely, materials like glass or fluoropolymers have low dielectric loss, absorb very few microwaves, and allow them to pass through. Such materials are difficult to heat with microwaves and are sometimes used as insulating material or windows.
2. Heat Generation and Transfer
A key characteristic of microwave heating is that heat is generated directly within the substance. Therefore, heat transfers from the inside outwards, fundamentally differing from conventional methods where heat is transferred “from the external surface inwards.” This difference allows for rapid heating of the core of the reaction system. It enables the formation of localized hotspots (high-temperature regions), which can be actively utilized for controlling selective reactions.
However, achieving uniform microwave irradiation within a container or powder bed in large-scale equipment is technically challenging. Incorrect wavelength selection, power adjustment, or equipment geometry design can lead to uneven heating or overheating, significantly reducing energy efficiency.
Consequently, recent efforts focus on improving heating uniformity through visualization of electromagnetic field distribution using numerical simulations and the introduction of phase control techniques using multiple antennas [12]. These advancements are expected to enhance the reliability and reproducibility of microwave heating in industrial applications.
3. Challenges in Heat Generation
While microwave heating generates heat extremely rapidly, some materials absorb microwaves poorly, making heating difficult. In such cases, measures like adding susceptors (microwave absorbers) with high dielectric loss or designing the reaction system to enhance overall heating efficiency are required.
Furthermore, due to the very high heating speed, safe management of the reaction system, including preventing thermal runaway and hotspot formation, becomes a critical issue. Essential elements include electromagnetic shielding design to prevent microwave leakage outside the equipment and operational know-how, such as real-time monitoring of heating behavior.
Technologies addressing these challenges are gradually being established, and with this foundation in place, microwave heating is moving beyond the laboratory scale towards full-scale implementation in industrial settings.
Microwave Applications in Chemical Reactions
1. Representative Chemical Reactions and Advantages
Microwave heating is applied to various chemical reactions, with notable effects observed particularly in organic synthesis (e.g., synthesis of pharmaceutical intermediates), polymerization and modification of polymer materials, and sintering of inorganic materials (e.g., ceramics). Applying microwaves to these reactions offers the following advantages:
- Rapid Reaction Completion: High heating responsiveness significantly shortens the overall reaction process time, enabling reduced manufacturing costs and rapid scale-up.
- Suppression of Side Reactions through Precise Temperature Control: When combined with cooling mechanisms, reaction temperature can be adjusted instantaneously, facilitating selective progression of the desired reaction.
- Improved Heating Uniformity and Product Quality: Internal heating creates a uniform temperature distribution, suppressing variations in product characteristics like molecular weight distribution or particle size. This aspect is central to the advancement of microwave chemistry.
2. Sintering Reactions in the Inorganic Materials Field
The sintering process for inorganic materials, including ceramics, is a promising application area for microwave heating. Conventional sintering methods require gradual heating over extended periods in high-temperature furnaces to achieve uniform sintering. In contrast, microwave sintering allows reaching high-temperature ranges from hundreds to thousands of degrees Celsius in a short time.
This rapid heating characteristic has been reported to yield benefits such as significant reduction in heating time, decreased energy consumption, and even suppression of excessive crystal grain growth [13]. Consequently, it may positively influence the microstructural control and mechanical strength of ceramics, leading to increased microwave adoption in materials engineering.
3. Microwave-Assisted Catalytic Reactions
Microwaves are gaining attention as an effective means of activating catalytic reactions. For instance, when ceramics or activated carbon supporting metal catalysts are heated with microwaves, localized hotspots (high-temperature regions) are reported to form on the catalyst surface, thereby promoting the reaction [14].
In addition to the heating effect of microwave irradiation, it has been suggested that interactions with the electric field component might influence the orientation and polarization state of reactive molecules. These factors are thought to contribute to improvements in reaction selectivity and efficiency.
Thus, by appropriately designing and controlling the complex physical effects induced by microwaves, advanced control over catalytic reactions that were difficult to realize with conventional thermal methods may become possible.
Accelerating Chemical Reactions with Microwaves
1. Primary Reasons for Acceleration
The acceleration of chemical reactions by microwave irradiation can primarily be attributed to the following three factors:
- Rapid Heating: Microwave heating generates heat directly within the target substance, leading to a speedy ramp-up to reaction temperature, significantly shortening the overall reaction time.
- Uniform Temperature Distribution: Unlike external heating, internal heating produces less temperature non-uniformity and a more homogeneous reaction field. Such conditions enable efficient reaction progression while suppressing side reactions caused by local overheating.
- Interaction with Electric Field (Potential Non-Thermal Effects): It has been suggested that the electric field component of microwaves influences molecular orientation and energy levels, potentially promoting reactions beyond simple heating effects. While these “non-thermal effects” are still under investigation, their clarification is anticipated in the future. Understanding these is vital for chemistry microwave research.
2. Experimental Data Examples
The effectiveness of microwave heating has been experimentally verified in various chemical reactions, with numerous reports of reduced reaction times and improved yields, particularly in organic synthesis and polymer polymerization reactions [15].
For example, in the polymerization of acrylonitrile, one case reported that microwave heating shortened the reaction time and increased the product yield compared to conventional methods [16]. Such results demonstrate that the reaction-promoting effects of microwave irradiation are being confirmed with reproducibility at the laboratory level.
If these findings are proven applicable on an industrial scale in the future, expectations for microwaves as a technology capable of achieving both process intensification and energy savings will further increase.
Organic Synthesis Utilizing Microwaves
1. Advantages in Organic Synthesis
Organic synthesis often involves multiple consecutive reaction steps, where temperature management and suppression of side reactions significantly impact synthesis yield and selectivity. Therefore, precise control of energy supply is required.
Microwaves can deliver energy rapidly and selectively to specific chemical species. Delivering energy facilitates control over the formation and decomposition of active intermediates, often leading to improved yield and purity of the target product, as reported in numerous cases. For instance, one research group successfully reduced a multi-step organic synthesis, typically requiring over two days, to less than a day using microwave irradiation, achieving the target compound in high yield [17].
2. Future Prospects
Microwave heating technology is expected to expand its applications further through integration with continuous synthesis (flow chemistry). For example, systems that incorporate microwave antennas into reaction flow paths are being developed, enabling continuous synthesis while monitoring temperature and reaction progress in real-time.
Such integration allows for optimization of reaction conditions and process stabilization, accelerating research towards realizing highly efficient and reproducible organic synthesis processes. The pharmaceutical industry, in particular, shows high interest in this approach, which offers both scalability and quality control.
Mechanism of Selective Heating and Advantages in Chemistry
1. Principle of Selective Heating
Microwaves leverage the differing dielectric permittivity and dielectric loss factors of substances to enable selective heating of specific components. This is particularly effective when the desired reactants or solvents in a reaction system strongly absorb microwaves, while other components absorb very little. For example, polar solvents and ionic compounds efficiently absorb microwaves and heat up, whereas non-polar molecules or components that are highly transparent to microwaves remain largely unheated. This allows concentrated energy delivery only to the necessary components, leading to expected effects like suppressing side reactions and avoiding unnecessary degradation due to heating.
Such “selective heating” is one of the major characteristics distinguishing microwave heating from conventional methods and is a key factor in dramatically enhancing reaction controllability.
2. Suppression of Unwanted Side Reactions
In conventional heating processes, prolonged exposure of the reaction mixture to high temperatures promotes the formation of undesired byproducts. Especially in reactions involving sensitive functional groups or intermediates, the duration of thermal exposure is often a primary cause of side reactions. In contrast, microwave heating rapidly reaches the reaction temperature, and the required reaction time is shortened, thereby reducing the opportunity for side reactions to occur, resulting in improved yield and selectivity. Furthermore, by utilizing the selective absorption characteristic of microwaves, it is possible to locally heat only the necessary compounds while minimizing thermal impact on surrounding components.
These characteristics are expected to contribute to simplifying purification processes and designing selective synthesis processes.
Utilization of Microwave Heating in Various Industrial Fields
1. Applications in Pharmaceutical Development
In pharmaceutical development, rapidly conducting complex organic synthesis with high reproducibility is crucial. As previously mentioned, microwave heating enables both reaction acceleration and high selectivity, making it a promising tool for developing new drugs and improving existing product processes.
Indeed, initiatives are underway to significantly shorten the lead time from prototyping to quality evaluation by introducing microwaves into multi-step synthesis reaction flows, thereby speeding up time-to-market.
The section ” Utilizing Microwave Technology in Pharmaceutical Development ” will detail specific examples of use and technological benefits in the pharmaceutical field. It will focus not only on optimizing synthesis reactions but also on the potential applications of microwaves in cutting-edge technology areas such as drug delivery systems (DDS) and continuous flow synthesis processes.
2. Food Industry
In the food industry, microwave heating technology contributes to process innovation in areas like sterilization, drying, and thawing. For instance, research is progressing on technologies that introduce microwaves into the heat sterilization process for retort foods, aiming to shorten processing times while preserving flavor and texture for long-term storage. Some manufacturing lines are already implementing this [18].
Efforts are also expanding towards rapid and uniform thawing of frozen foods and applications in temperature control processes, aiming for overall efficiency improvement and energy savings in cooking processes. Future applications, such as product-optimized heating leveraging the dielectric properties of specific foods, are also anticipated.
3. Materials Science
Technology utilizing microwave heating to efficiently cure composite materials such as carbon fiber reinforced plastics (CFRP) is attracting attention in materials science. Since microwaves also act on highly conductive materials, they enable uniform heating from within the material, promising reduced curing times and improved energy efficiency.
This technology is expected to contribute to weight reduction and manufacturing cost reduction for structural materials in the aerospace and automotive sectors, with demonstration studies and pilot line applications already reported [19].
4. Energy and Environmental Fields
In the energy and environmental fields, microwave heating is recognized as a promising technology contributing to resource circulation and the realization of a decarbonized society. For example, the conversion of waste plastics into hydrogen and carbon materials using microwaves allows for the production of high-value substances without using fossil fuels, holding promise for both CO₂ emission reduction and resource recycling [20].
Furthermore, applications are expanding to include rapid drying and carbonization of sewage sludge and food waste, and selective decomposition of difficult-to-decompose composite materials (like FRP), drawing attention as a technology for waste volume reduction and recycling.
Additionally, efforts are underway to utilize surplus electricity from renewable energy sources to convert it into chemical energy using microwaves (Power-to-Chemical), raising expectations from the perspectives of energy storage and supply-demand adjustment.
Utilizing Microwave Technology in Pharmaceutical Development
1. Contribution to Synthesis and Quality Improvement
In pharmaceutical development, the ability to synthesize target molecules rapidly and with high yield and purity is a critical factor influencing development efficiency. Microwave heating has been reported to not only dramatically increase reaction rates but also contribute to the suppression of byproducts, making it effective for purifying synthetic intermediates [21].
Indeed, examples have been reported where applying microwaves to the synthesis processes of anticancer drugs and antibiotics has simplified complex reaction pathways, reduced synthesis steps, and improved yields. Such application holds high potential for shortening the development period from preclinical stages to scale-up, and its adoption in the process development sites of pharmaceutical companies is progressing.
2. Application to Drug Delivery Systems (DDS)
Microwave technology is also finding applications in the field of Drug Delivery Systems (DDS), which has garnered significant attention recently. In DDS, precise structural control of nanoparticles and microcapsules is crucial for efficiently delivering drugs to specific sites within the body.
Microwave heating is considered effective for optimizing drug encapsulation rates and particle size distribution because it allows for rapid and uniform control of particle nucleation and growth processes. Research into synthesis techniques leveraging this advantage is advancing.
Specifically, attempts to apply microwaves to nanoparticle synthesis under continuous flow conditions have begun in some corporate research labs, raising expectations for achieving both reproducible manufacturing and scalability.
3. Future Vision for Pharmaceutical Factories
The pharmaceutical manufacturing field currently envisions the realization of “modular flow factories” that integrate the entire process from reaction to purification and final formulation into a continuous flow [22]. In this approach, microwave heating, as a means of rapid and selective reaction control, is highly compatible and expected to contribute to the overall optimization of the process.
If modular continuous production is realized, it will enable improved productivity, consistent quality, and reduced manufacturing costs compared to conventional batch methods. It will also lead to a shorter time-to-market for new drugs. While still partially in the demonstration phase, pilot implementations are underway at major pharmaceutical companies in Europe and the US, suggesting it could potentially become the standard model for the pharmaceutical industry in the future.
Industrial Scale Applications of Microwave Technology
1. Operational Examples in Large-Scale Factories
Even in Japan, continuous chemical product manufacturing processes utilizing microwave heating technology are entering the demonstration phase. For example, at our company’s demonstration facility (Microwave Chemical Co., Ltd.) located in Suminoe-ku, Osaka, verification of continuous reaction systems using microwaves is being conducted, reporting energy savings, space savings, and shorter processing times compared to conventional reaction vessels [23].

This facility also conducts process contract testing and scale-up studies for multiple chemical manufacturers, accumulating concrete results towards the industrialization of microwave technology.
2. Equipment Challenges and Technological Innovation
In large-scale factories, it is necessary to combine multiple high-output microwave generators (magnetrons or solid-state amplifiers), feed them into a shielded furnace, and process large quantities of raw materials simultaneously. Controlling electromagnetic wave interference and reflection, and designing for uniform heating are critical challenges. In recent years, technologies combining microwaves of multiple frequency bands or dynamically changing the phase have been researched and commercialized, enabling relatively uniform heating even on an industrial scale.
3. Expectations for Large-Scale Implementation
As microwave heating technology is introduced into large-scale plants in earnest, significant improvements in energy efficiency compared to conventional heating and pyrolysis processes are expected. Demonstration cases have reported energy savings of up to approximately 50%, which is anticipated to lead to reduced running costs during mass production.
Additionally, process designs capable of handling diverse unused resources like biomass and waste plastics collectively are advancing. Microwave technology is considered increasingly important as a core technology for achieving a resource-circulating society and chemical recycling [24].
The Future of Microwave Heating and Technological Innovation
Latest Trends in Evolving Microwave Heating Technology
1. Semiconductor Devices
While magnetrons, like those in microwave ovens, were traditionally common, high-output oscillators using semiconductor devices such as GaN (Gallium Nitride) have recently been developed. Microwave irradiation equipment capable of precisely controlling frequency and phase is emerging [25]. Such capability is expected to enable real-time control of wave interference inside the equipment, potentially minimizing heating non-uniformity to the extreme.
2. International Trends
Overseas, the industrial application of microwave heating technology is also rapidly advancing. For example, SAIREM in France has developed a continuous plant extraction process using microwaves, which is already being implemented on an industrial scale [26]. This technology offers multiple benefits, including reduced processing time, lower solvent consumption, and high-yield extraction, successfully achieving both CO₂ emission reduction and production efficiency.
Furthermore, in Asian regions like China and Korea, unique microwave technologies are being deployed, primarily in the fields of carbon materials and ceramics processing, with commercial-level results reported. Amidst this, Japanese companies leverage their unique strengths to lead international competition across multiple technology areas, including materials design, equipment control, and process integration.
Challenges and Limitations of Microwave Heating
1. Technical Constraints
One of the biggest technical challenges in microwave heating technology is ensuring heating uniformity on a large scale. While controlling electromagnetic wave distribution is relatively easy at the laboratory level, at the plant level, reflections and interference can cause “hotspots,” leading to risks such as localized overheating or reaction runaway/stoppage at the plant level.
Particularly when the material being processed is a multiphase system or powder, or when the reaction vessel design includes many metal components, phenomena like microwave reflection and localized discharge are more likely to occur, requiring caution regarding safety. Therefore, designing industrial equipment necessitates advanced expertise in integrating and optimizing multiple design factors, including electromagnetic field distribution, shielding, cooling, and safety measures.
2. Cost and Economics
Introducing microwave heating systems tends to involve high initial investment costs for oscillators, control equipment, and shielding facilities, which has been one bottleneck to widespread adoption. Specifically, the manufacturing and maintenance costs of high-power microwave equipment, and technological development towards longer lifespan and lower cost, remain challenges. Our company (Microwave Chemical Co., Ltd.) is actively working in this area.
Price reduction through mass production effects and optimization of operational costs is expected in the future. Indeed, companies are increasingly adopting the technology incrementally for specific applications, suggesting that cost concerns are steadily being addressed through technological evolution.
3. Heating Non-Polar Substances
Microwave heating is particularly effective for systems that can efficiently absorb microwaves, such as polar molecules or ionic substances. However, non-polar organic solvents and some polymer materials tend to have low microwave absorption, significantly reducing heating efficiency. When applying microwaves to such systems, countermeasures are taken, such as using absorption aids (susceptors) with high dielectric loss or optimizing the reaction system design to create structures that facilitate microwave energy transfer.
In recent years, methods to enhance the microwave responsiveness of the materials themselves (e.g., dispersing conductive microparticles) are also advancing, promising broader applicability to various reaction systems in the future.
Potential of Microwaves in a Sustainable Society
1. Environmental Load Reduction and Energy Efficiency
Microwave heating is considered a powerful means of contributing to environmental load reduction due to its higher energy efficiency than conventional external heating methods and ability to suppress unnecessary heat loss. Particularly in the current industrial environment where CO₂ emission reduction is strongly demanded internationally, energy saving is a major driver for adoption.
Furthermore, gasification and liquefaction technologies for waste plastics and chemical waste using microwaves are attracting attention as initiatives aiming to achieve both waste recycling and greenhouse gas emission control. They hold high expectations as next-generation technologies contributing to the realization of a resource-circulating society [24].
2. Collaboration with Renewable Energy
A challenge accompanying the widespread adoption of renewable energy sources like solar and wind power is the fluctuation in power output and instability in supply-demand balance. Microwave heating, with its ability for instant on-off control and extremely short startup time, is drawing attention as a technology that can flexibly utilize variable electricity derived from renewables.
Specifically, if Power-to-Chemical systems are realized—operating microwave heating during periods of surplus electricity to convert and store energy in chemical forms (e.g., syngas or liquid fuels)—they could significantly contribute not only to the efficient use of renewables but also to the construction of a decentralized energy society.
3. Regional Revitalization and Decentralized Processing
Since microwave equipment can often be designed compactly, it offers the advantage of being easily deployable not only in factories and large urban areas but also in decentralized, small-scale facilities such as local agricultural processing or waste treatment sites. Thus, it holds promise from the perspective of regional revitalization, enabling applications like small-scale biomass utilization, food processing, and ore calcination in remote areas or islands.
Conclusion
Microwaves, utilizing an energy transfer method via electromagnetic waves fundamentally different from conventional heating techniques, represent a technology with the potential to achieve acceleration, high efficiency, and improved selectivity in chemical reactions and material processing. Application examples leveraging internal and selective heating characteristics have already reported results such as energy saving effects, enhanced reaction rates, and improved product quality.
Particularly in the chemical industry, where many processes require high temperature and pressure conditions, the impact of process innovation through the introduction of microwave technology is considered extremely large.
While challenges such as ensuring heating uniformity, equipment costs, and safety management for large-scale applications exist, recent advancements in simulation, AI control, etc., are accelerating implementation to overcome these issues. Pilot plants and demonstration equipment are already operational domestically and internationally, making industrial implementation increasingly realistic.
Furthermore, regarding global societal challenges like carbon neutrality and resource circulation, microwave technology is expected to contribute in multiple ways, including its compatibility with renewable energy, suitability for decentralized production systems, and waste recycling.
Overall, microwave technology holds the potential to become a core next-generation process technology supporting the industrial structural transformation of the 21st century. Its research, development, and societal implementation trends remain important themes to watch closely in the future.
References
[1] Tsukahara, Yasunori. “Industrial Development of Microwave Chemistry.” Production and Technology, vol. 66, no. 1, 2014, pp. 20–25.
[2] The Japan Institute of Invention and Innovation. “100 Selections of Post-War Japanese Innovation High Economic Growth Period Microwave Oven.” The Japan Institute of Invention and Innovation Website. https://koueki.jiii.or.jp/innovation100/innovation_detail.php?age=high-growth&eid=00046&page=keii (Accessed: May 4, 2025)
[3] Sharma, A., & Sharma, G. (2017). “Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave Heating.” Micromachines, 8(7), 1–20.
[4] CEM Corporation. “Microwave Heating – Increasing Reaction Rate.” CEM.com. Website. https://cem.com/microwave-heating-increasing-reaction-ratecem.com+1Chemical & Engineering News+1 (Accessed: May 4, 2025)
[5] JapanGov. “The Microwave Technology Turning the Chemical Industry Green.” JapanGov. Website. https://www.japan.go.jp/kizuna/2024/02/the_microwave_technology.htmlJapanGov – The Government of Japan (Accessed: May 4, 2025)
[6] Nguyen, Thanh, Quoc Dat Nghiem, Dongpyo Kim, and Jong San Chang. “Microwave assisted synthesis of high molecular weight polyvinylsilazane via RAFT process.” Polymer, vol. 50, no. 21, 2009, pp. 5037–5041.
[7] Mitsui Chemicals, Inc. “Mitsui Chemicals, Microwave Chemical Complete Setup of Demonstration Facility for Innovative Microwave-Based Carbon Fiber Production Technology at Mitsui Chemicals’ Nagoya Works.” Mitsui Chemicals News Release, February 8, 2024.
[8] Antonio de la Hoz, Ángel Díaz-Ortiz, Andrés Moreno. “Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects.” Chemical Society Reviews, vol. 34, no. 2, 2005, pp. 164–178.
[9] Thanh Lieu, Suzana Yusup, Muhammad Moniruzzaman, “Microwave-assisted Esterification Reaction of Free Fatty Acids from Ceiba Pentandra Seed Oil,” Australian Journal of Basic and Applied Sciences, 9(30): 41–45, 2015.
[10] Kedafi Belkhir, Guillaume Riquet, Frédéric Becquart. “Polymer Processing under Microwaves” Advances in Polymer Technology 2022
[11] Yamada, S., et al. (2011). “Microwave-assisted low-temperature dehydration polycondensation of dicarboxylic acids and diols.” Polymer Journal, 43, 1003–1007.
[13] Morteza Oghbaei and Omid Mirzaee, “Microwave versus conventional sintering: A review of fundamentals, advantages and applications,” Journal of Alloys and Compounds, vol. 494, no. 1–2, pp. 175–189, 2010.
[14] Yifan Deng et al. “Microwave-assisted conversion of methane over H-(Fe)-ZSM-5: Evidence for formation of hot metal sites.” Chemical Engineering Journal, Vol. 420, Issue 15 September 2021; ISSN 1385-8947
[15] C. O. Kappe. “Controlled microwave heating in modern organic synthesis.” Angewandte Chemie International Edition, 2004, 43(46), pp. 6250–6284.
[16] Biswal, T., Samal, R., & Sahoo, P. K. (2010). Complex-mediated microwave-assisted synthesis of polyacrylonitrile nanoparticles. Nanotechnology, Science and Applications, 3, 77–83
[17] C. O. Kappe. “Microwave dielectric heating in synthetic organic chemistry.” Chemical Society Reviews, 2008, 37(6), pp. 1127–1139.
[18] Xue, Q., Xue, C., Luan, D., Wang, Y., Wen, Y., Bi, S., Xu, L., & Jiang, X. (2023). Unlocking the Potential of Microwave Sterilization Technology in Ready-to-Eat Imitation Crab Meat Production. Foods, 12(24), 4412.
[19] Alekajbaf, Y., Murali, S., & Dancila, D. (2024). Energy efficient microwave curing of carbon fiber reinforced polymer via metamaterial matching and advanced electromagnetic exposure control. International Journal of Microwave and Wireless Technologies, 16(10), 1671–1677.
[20] Jie, X., Li, W., Slocombe, D., Gao, Y., Banerjee, I., AlMegren, H. A., Alshihri, S., Dilworth, J. R., & Xiao, T. (2020). Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nature Catalysis, 3(11), 902–912.
[21] Pal, A., & Gayen, K. S. (2021). The Impact of Microwave Irradiation Reaction in Medicinal Chemistry: A Review. Oriental Journal of Chemistry, 37(1).
[22] Mascia, S., Heider, P. L., Zhang, H., Lakerveld, R., Benyahia, B., Barton, P. I., Braatz, R. D., Cooney, C. L., Evans, J. M. B., Jamison, T. F., Trout, B. L. (2013). End-to-End Continuous Manufacturing of Pharmaceuticals: Integrated Synthesis, Purification, and Final Dosage Formation. Angewandte Chemie International Edition, 52(47), 12359–12363.
[23] Microwave Chemical Co., Ltd. (May 29, 2024). Completion of Continuous Demonstration Unit for Chemical Recycling Using Microwaves, Promoting our Focus on Standardization Business Website. https://mwcc.jp/news/3475/(Accessed: May 6, 2025)
[24] NEDO (New Energy and Industrial Technology Development Organization). (November 1, 2022). Japan’s First Completion of Large-Scale General-Purpose Demonstration Facility for Chemical Recycling Technology Using Microwaves ―Contributing to the Realization of a Circular Economy through the Recycling of Waste Plastics―. Website. https://www.nedo.go.jp/news/press/AA5_101587.htmlNEDO+1NEDO+1 (Accessed: May 6, 2025)
[25] Ikeda, H., & Itoh, Y. (2019). A 2.4 GHz-Band 250 W, 60% Feedback-Type GaN-HFET Oscillator Using Imbalanced Coupling Resonator for Use in the Microwave Oven. Applied Sciences, 9(14), 2887.
[26] SAIREM. (2021). Industrial scale continuous microwave-assisted plants extraction. Website. https://www.sairem.com/industrial-scale-continuous-microwave-assisted-plants-extraction/ (Accessed: May 6, 2025)

