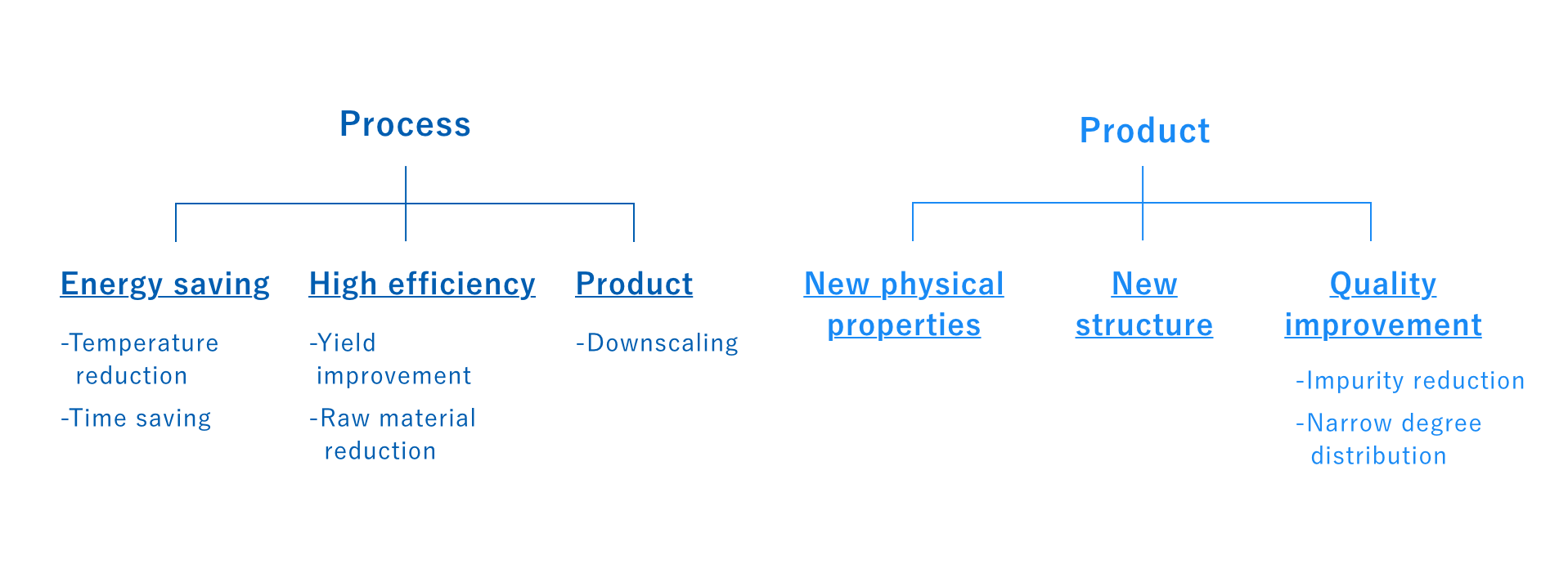
Flow – 02
Laboratory Verification
The next step in reaction system design is choosing equipment that will create the conditions specified in the previous section. Equipment is selected based on the target reaction system (liquid phase, solid phase (powder, slurry, film, etc.), gas-solid, drying, distillation) and microwave absorption characteristics (complex dielectric constant ε”) to meet various conditions such as microwave energy input, electromagnetic field distribution, frequency, temperature, pressure, etc.
In addition to commercially available equipment, we also design and manufacture our own equipment for a wide range of reaction types and conditions. Applications include general liquid-phase synthesis reactions (decompressed or pressurized), stirring (kiln, impeller, stirrer), heating or drying, and calcination at temperatures in excess of 1000°C. Efficient heating can be achieved by selecting either electric or magnetic field heating according to the microwave absorption characteristics of the heated object.
-
Egg-One
-
Microwave Solid Phase Synthesizer
Microwave Effects
The final step involves optimizing reaction conditions. We clarify the advantages of microwaves against other competing methods and heat sources and obtain data that will contribute to the design of the final industrial-scale machine.
The effects obtained from using microwaves as an energy source will vary depending on the target but will most likely fall into one of the categories below. For example, we’ve seen many reactions achieve reduced processing time, impurities, and lower temperatures, and have even seen examples of reactions being activated that would not otherwise occur with conventional heating. In some cases, the synthesized product may also exhibit special characteristics, such as with metal nanoparticles, silver nanowires, or zeolites. Selective energy transfer and heating can also often provide improved quality that can inhibit coloration and other transformations.
Reaction Optimization(Catalyst Synthesis and Design)
Microwave absorption capacity is a material eigenvalue, and catalysts are no exception. However, we have highly flexible catalyst design technology that is not limited by the microwave absorption capacity of the catalyst. Even if the catalyst to be developed does not absorb microwaves easily, we can create a composite catalyst with a support that readily absorbs microwaves.
Catalyst Design Technology
We use the following three elemental technologies to flexibly design composite catalysts for the catalyst molecules to be developed.
- Analysis of electrical loss, magnetic loss, and conductive loss as indicators of microwave absorption capacity
- Analysis of decomposition temperature characteristics of adsorbed molecules
- Evaluation of specific surface area and porous distribution
Our catalyst library, which we have accumulated through years of research and development, now includes more than 50 catalysts for multiple chemical reactions.
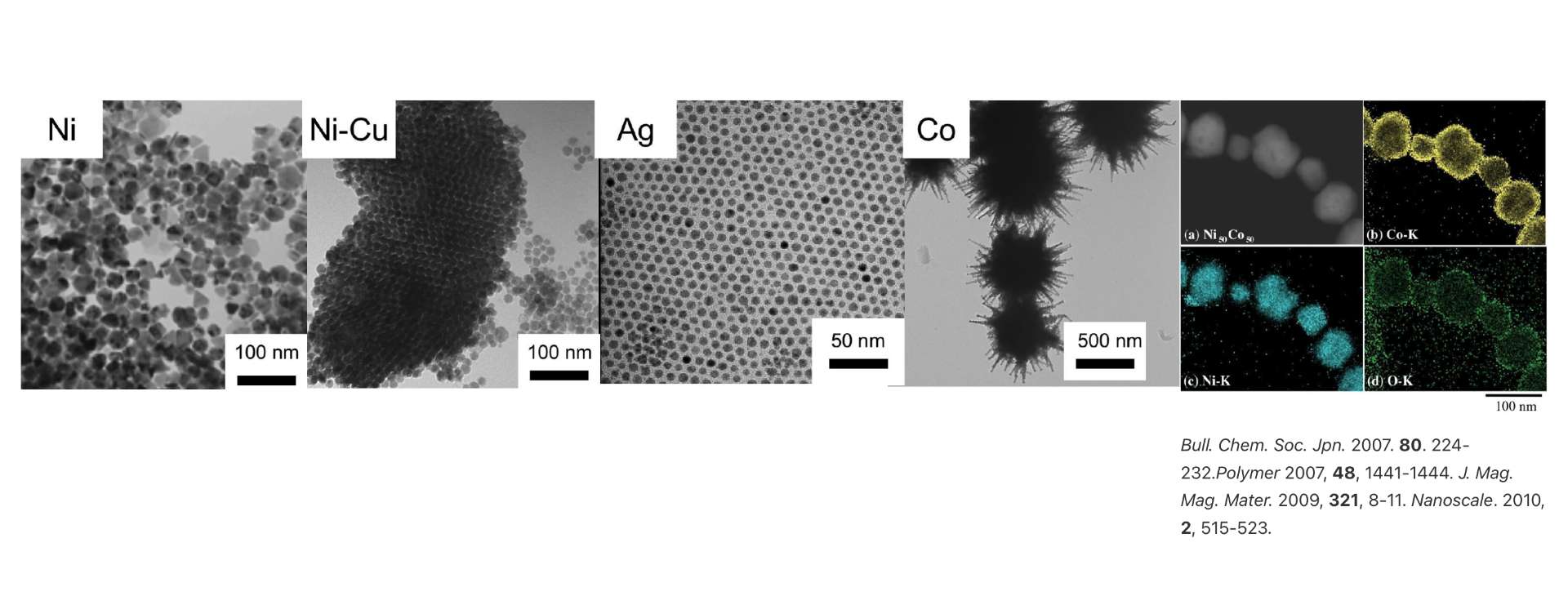
Catalysts are important materials that are widely used in industrial applications such as chemical manufacturing as well as environmental applications such as vehicle exhaust. We have created more advanced and efficient catalytic reactions through the use of microwave.
Specifically, microwave selectively heats the reaction site – either the catalyst or an area near the catalyst – thereby reducing the overall reaction temperature in the reactor and extending catalyst life.
In addition to the development of equipment and processes that enables selective microwave heating of catalysts, we are also working on the following projects for the development of key technologies for catalyst preparation.
(i) synthesis of nano catalyst particles
(ii) design of catalysts suitable for microwave heating
In the process of synthesizing nano catalyst particles, the uniform and selective heating capabilities that are the special features of microwave heating makes it possible to produce nano-scale particles more efficiently and economically, compared to conventional heat transfer. This is because, while conventional heat transfer may cause temperature gradients in the reactor, microwave heating allows more uniform nucleation and the narrower particle distribution. Using a dispersing agent with a high microwave absorption capacity to suppress particle agglomeration allows us to selectively heat said agent and alter the shape of the nanoparticles.
Particularly with the catalysts for bulk chemicals, even a small increase in yield significantly increases economic value but using nano catalyst particles to enlarge the specific surface area of the reaction can be expected to increase reaction efficiency and speed.
Microwave filler (microwave absorber)






